Poor Prognosis in GI Cancers

Patients with advanced GI cancers need innovation
Diverse GI cancers—including gastric, GEJ, esophageal, and biliary tract—present a range of challenges. They’re heterogeneous and complex, and most patients have advanced disease at diagnosis.1,2
Nearly 50,000 cases of gastroesophageal adenocarcinoma (GEA) are diagnosed yearly across the US in patients with a median age of 68. Incidence is higher among non-white populations and is particularly increasing among younger patients. Biliary tract cancers are more rare, with ~15,000 new cases diagnosed each year in the US.3-6
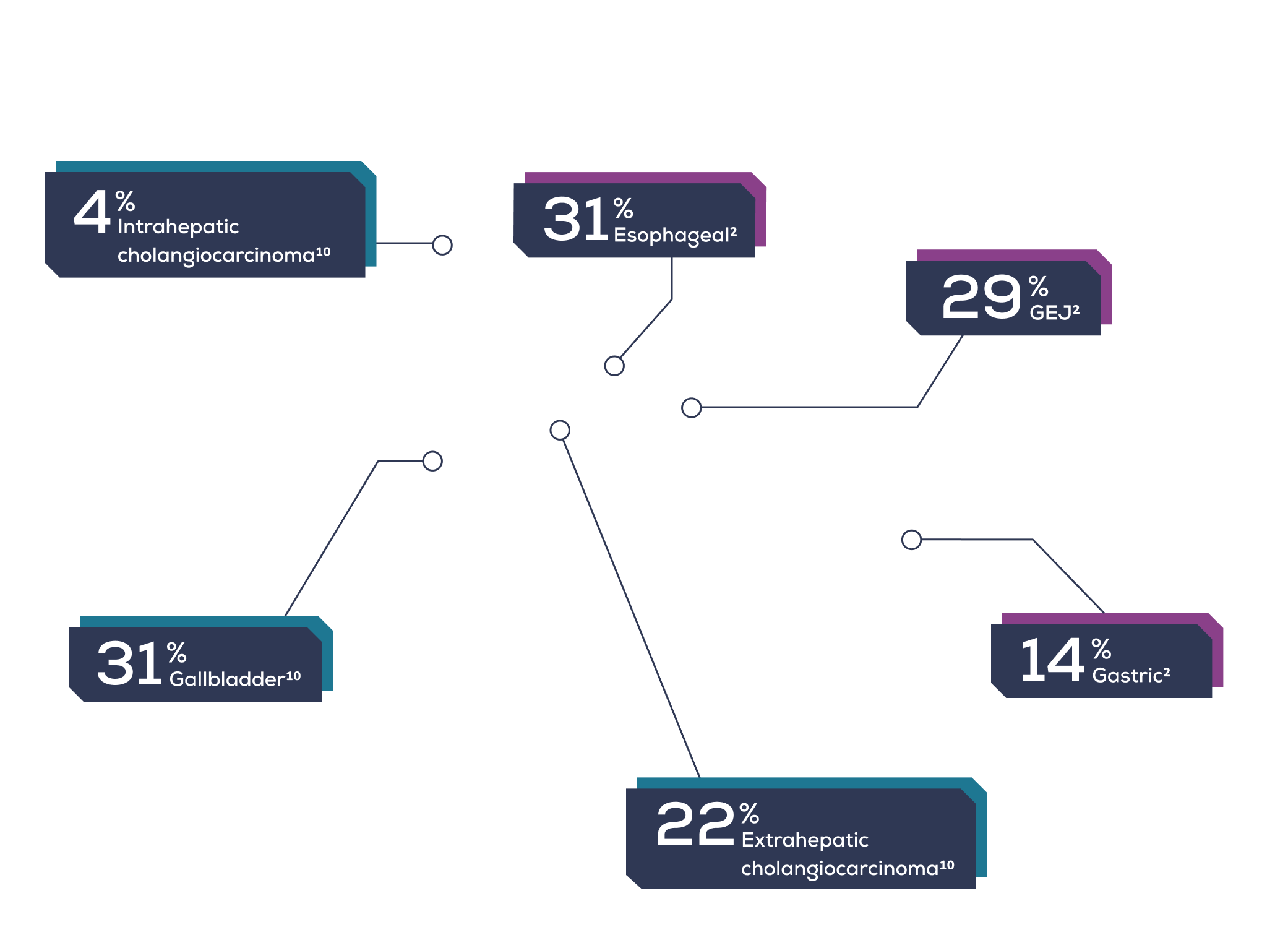
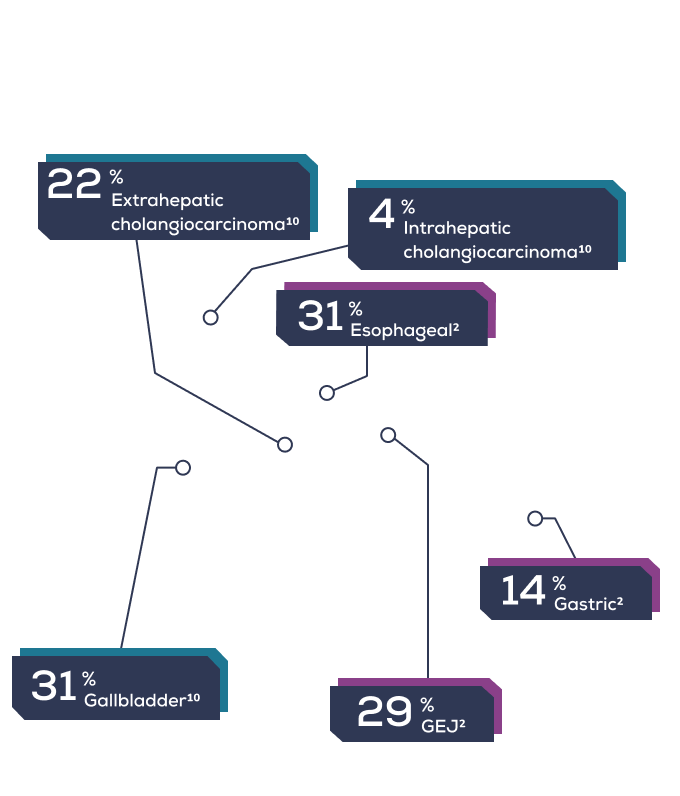
Presentation by cancer subtype
- ESOPHAGEAL
- GASTRIC
- GEJ
- GALLBLADDER
- EXTRAHEPATIC CHOLANGIOCARCINOMA
- INTRAHEPATIC CHOLANGIOCARCINOMA
These cancers are typically aggressive and difficult to treat. Patients face a dismal prognosis despite current treatment options.1,2
5-year survival rate for metastatic disease
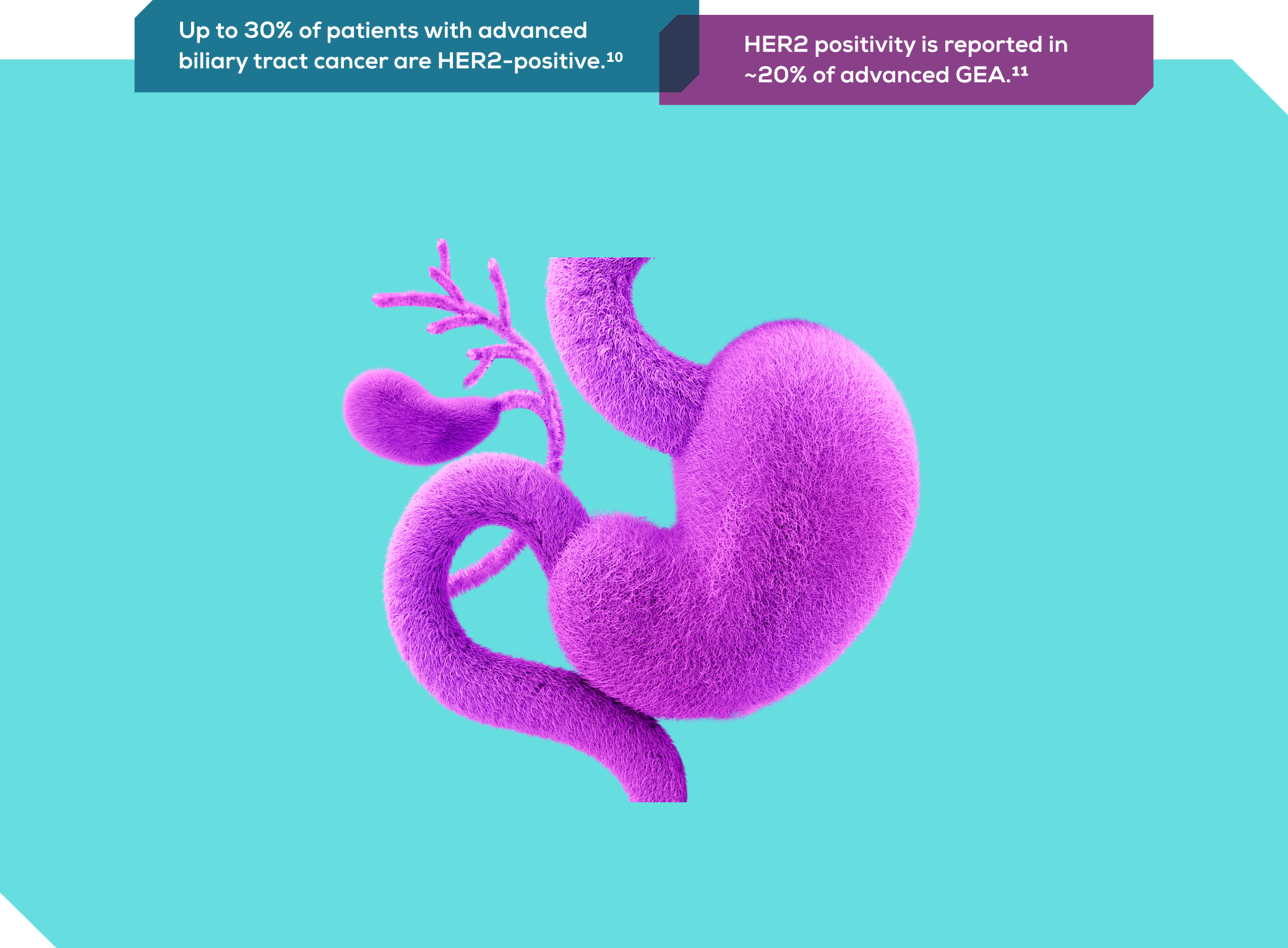
HER2 is a proven and actionable biomarker
HER2 was the first biomarker used to guide clinical decisions in advanced GEA. It is an actionable biomarker for targeted treatment in GEA and biliary tract cancer.9

More than incremental clinical advancements are needed in GEA
Despite the approval of the combination of trastuzumab, chemotherapy, and pembrolizumab for HER2-positive and PD-L1–positive patients, there hasn’t been a HER2-targeted innovation for 1L treatment since trastuzumab was approved in 2010.12










AN UPGRADE IS NEEDED IN HER2-TARGETED 1L TREATMENTS TO IMPROVE OUTCOMES FOR ADVANCED HER2+ GEA
*Data are from a retrospective observational cohort study using a Flatiron Health EHR-derived de-identified database that included 942 patients diagnosed with adenocarcinoma in the US.2
†ToGA was an open-label, international, phase 3, randomized controlled trial that investigated trastuzumab in combination with capecitabine plus cisplatin or fluorouracil plus cisplatin against chemotherapy alone in 594 patients with HER2-positive advanced gastric or GEJ adenocarcinoma. Primary endpoint was OS.13
‡Keynote 811 was an international, randomized, double-blind, phase 3, placebo-controlled trial that investigated pembrolizumab against placebo in combination with the SOC, which was trastuzumab and either cisplatin and 5-fluorouracil or oxaliplatin and capecitabine, in 698 patients with HER2-positive advanced gastric or GEJ adenocarcinoma. Dual primary endpoints were progression-free survival and OS.14,17
§LOGiC was an international, multicenter, double-blind, randomized, phase 3 trial that investigated lapatanib against placebo in combination with capecitabine and oxaliplatin in 545 patients with HER2-positive advanced GEA. Primary endpoint was OS.15
¶JACOB was a double-blind, placebo-controlled, randomized, multicenter, phase 3 trial that investigated pertuzumab against placebo in combination with trastuzumab and either cisplatin and capecitabine or 5-fluorouracil in 780 patients with HER2-positive metastatic gastric or GEJ adenocarcinoma. Primary endpoint was OS.16
1L=first-line; EHR=electronic health record; GEJ=gastroesophageal junction; GI=gastrointestinal; HER2=human epidermal growth factor receptor 2; OS=overall survival; PD-L1=programmed death ligand 1; SOC=standard of care.
1. Kim H, Kim R, Kim HR, et al. HER2 aberrations as a novel marker in advanced biliary tract cancer. Front Oncol. 2022;12:834104. doi:10.3389/fonc.2022.834104 2. Mehta R, Liepa AM, Zheng S, Chatterjee A. Real-world molecular biomarker testing patterns and results for advanced gastroesophageal cancers in the United States. Curr Oncol. 2023;30(2):1869-1881. doi:10.3390/curroncol30020145 3. Cytryn SL, Janjigian YY. HER2 targeting in esophagogastric cancer: redefining the landscape and breaking barriers. J Natl Compr Canc Netw. 2023;21(4):423-429. doi:10.6004/jnccn.2023.7010 4. Cancer Stat Facts: Stomach Cancer. Accessed April 15, 2025. https://seer.cancer.gov/statfacts/html/stomach.html 5. Cancer Stat Facts: Esophageal Cancer. Accessed April 15, 2025.